Abstract
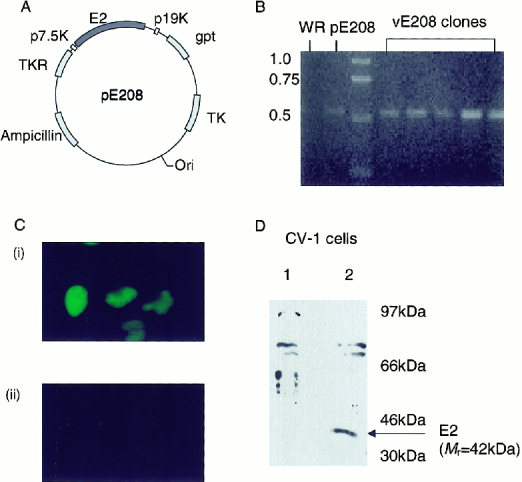
Intratypic diversity of the human papillomavirus (HPV) type 16 Recombinants genome is generally characterized by a point mutation, insertion, and/or deletion. Using PCR-based cloning and sequencing, we detected concurrent infection with 8 HPV16 variants in a woman enrolled in the ASCUS-LSIL classification study. The European variant was the main variant; each of the 7 minor variants had partial DNA sequences identical to the European variant and another part identical to the African variant 2. At a follow-up visit, only one African variant 2 of HPV16 was detected. The results of the present study suggest the presence of intratympanic recombination of the HPV genome in natural infection.
Bacterial culture strains and conditions.
The strain used in this study was L. casei CECT 5275 [=ATCC 393(pLZ15-)]. Wild-type L. casei was grown in MRS medium (Difco) at 37°C without shaking. For expression analysis, recombinant L. casei was cultured in basal MRS medium (10 g peptone per litre, 8 g meat extract per litre, 4 g yeast extract per litre, 2 g monobasic potassium phosphate per litre). litre, 5 g sodium acetate per litre, 2 g diammonium citrate per litre, 0.2 g magnesium sulfate per litre, 0.03 g manganese sulfate per litre, 1 mL Tween 80 per litre, buffered with 0.2 M potassium phosphate [pH 7.0]) supplemented with 0.5% lactose as a carbon source for induction and with 0.5% glucose as a carbon source for repression of the operon promoter. lactose from L. casei. Escherichia coli DH5α was grown in LB medium at 37°C with shaking for replication of expression vectors in cloning procedures. The erythromycin concentrations used for the selection of recombinant strains were 350 μg/ml and 5 μg/ml for E. coli and L. casei, respectively.
Electron microscope.
L. casei and L. casei/L1 were grown until the OD600 was 2. Cultures (2 ml) were centrifuged at 5000 × g for 10 min and each pellet was washed three times in saline. Bacteria were fixed with 3% paraformaldehyde (Sigma) for 1 hour at room temperature. Appropriate volumes of fixed intact bacteria were adhered to carbon-coated grids for 1 min and air-dried. Some samples were negatively stained with 2% uranyl acetate for 1 min. Samples were examined with a Zeiss EM 109 transmission electron microscope operated at 80 kV.
Immunofluorescence.
L. casei and L. casei/L1 were washed and fixed as described above. Cells then adhered to polylysine-coated microscopic glass slides. To detect VLPs produced by L. casei by immunofluorescence, slides were incubated with an anti-HPV-16 VLP conformational antibody, H16.V5 (10) (kindly provided by N. Christensen, Department of Microbiology and Immunology, Pennsylvania State University College of Medicine, Hershey, PA), diluted in PBS containing 0.01% Tween 20 and 0.5% BSA for 2 h at 37 °C.
Cells were then washed five times in PBS for 5 minutes and incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (Sigma) diluted in PBS containing 0.01% Tween 20 and 1% BSA. 5% for 1 hour at 37°C. After five washes in PBS, slides were air-dried, embedded in Mowiol (Calbiochem), covered with coverslips, and stored at 4°C until use. Fluorescence was visualized by exposing slides to a filter with an excitation wavelength of 488 nm from a Zeiss LSM 510 META confocal microscope.
Immunization of mice.
Six to eight-week-old female BALB/c mice from the Butantan Institute were used for subcutaneous immunization experiments. L. casei and L. casei/L1 were cultured until the OD600 was 2. The bacteria were collected by centrifugation at 5000 × g and washed three times with non-pyrogenic saline, and then the concentration was adjusted to 109 cells per 100 μl.
Groups of five mice were inoculated with 100 µl of saline, L. casei or L. casei/L1 on two consecutive days. Three administrations, one sensitizing and two boosting, were performed at 2-week intervals, for a total of six administrations. Ten days after the last administration, animals were bled through the retroorbital plexus and pooled sera were collected and stored at -20°C until use. The results described below are representative of two experiments.
ELISA for the detection of anti-HPV-16 VLP antibodies.
ELISA plates were coated with 100 ng of VLPs derived from S. frugiperda insect cells diluted in PBS (assembled VLPs) or 0.2 M carbonate buffer (pH 9.6) (disassembled VLPs) (23) at 4 °C for 16 h. Pooled sera were tested for the presence of anti-L1 IgG as previously described (1). The titer was defined as the dilution at which the absorbance at 492nm was 0.15. The results presented below are the means of two independent immunization experiments.